- Research
- Open access
- Published:
The effectiveness of case management for cancer patients: an umbrella review
BMC Health Services Research volume 22, Article number: 1247 (2022)
Abstract
Background
Case management (CM) is widely utilized to improve health outcomes of cancer patients, enhance their experience of health care, and reduce the cost of care. While numbers of systematic reviews are available on the effectiveness of CM for cancer patients, they often arrive at discordant conclusions that may confuse or mislead the future case management development for cancer patients and relevant policy making. We aimed to summarize the existing systematic reviews on the effectiveness of CM in health-related outcomes and health care utilization outcomes for cancer patient care, and highlight the consistent and contradictory findings.
Methods
An umbrella review was conducted followed the Joanna Briggs Institute (JBI) Umbrella Review methodology. We searched MEDLINE (Ovid), EMBASE (Ovid), PsycINFO, CINAHL, and Scopus for reviews published up to July 8th, 2022. Quality of each review was appraised with the JBI Critical Appraisal Checklist for Systematic Reviews and Research Syntheses. A narrative synthesis was performed, the corrected covered area was calculated as a measure of overlap for the primary studies in each review. The results were reported followed the Preferred reporting items for overviews of systematic reviews checklist.
Results
Eight systematic reviews were included. Average quality of the reviews was high. Overall, primary studies had a slight overlap across the eight reviews (corrected covered area = 4.5%). No universal tools were used to measure the effect of CM on each outcome. Summarized results revealed that CM were more likely to improve symptom management, cognitive function, hospital (re)admission, treatment received compliance, and provision of timely treatment for cancer patients. Overall equivocal effect was reported on cancer patients’ quality of life, self-efficacy, survivor status, and satisfaction. Rare significant effect was reported on cost and length of stay.
Conclusions
CM showed mixed effects in cancer patient care. Future research should use standard guidelines to clearly describe details of CM intervention and its implementation. More primary studies are needed using high-quality well-powered designs to provide solid evidence on the effectiveness of CM. Case managers should consider applying validated and reliable tools to evaluate effect of CM in multifaced outcomes of cancer patient care.
Background
Cancer ranks as one of the leading causes of premature death among population around 30–69 years old across 134 countries [1], and the global incidence of cancer is about to reach 30.2 million new cases and 25.7 million deaths by 2040 [2]. Earlier detection and diagnosis, and development of diverse cancer treatments have increased the survival rate of cancer patients. According to Quaresma et al. [3], the cancer survival in the UK has doubled over the last 40 years alongside the advancement in cancer diagnosis and treatment. However, number of challenges exist in the current cancer care all over the world. Many cancer patients oftentimes receive a series of long-running and exhausting multi-modal treatments and experience descent in psychological, physical and social functioning, which have a significant negative impact on their quality of life (QoL) [4, 5]. In addition, the significant healthcare spending and productivity losses of cancer patients lead to a heavy patient economic burden, which is another substantial issue with cancer care [6]. A systematic approach is needed to mobilize and deliver appropriate resources, provide accessible, safe, and well-coordinated care for cancer patients received stressful treatments and shouldered heavy economic burden [7].
Case management (CM) is defined by the Case Management Society of America (CMSA) as “a collaborative process of assessment, planning, facilitation, care coordination, evaluation, and advocacy for options and services to meet an individual’s and family’s comprehensive health needs through communication and available resources to promote quality, cost-effective outcomes” (P. 11) [8]. According to the definition, CM is designed to use resources effectively to improve the quality of treatments, patient care services, and QoL of patients while reducing the relevant healthcare costs.
With the worldwide utilization of CM in cancer patient care, studies examining the effect of CM in improving patient-related outcomes or healthcare service use outcomes have been skyrocketing. Numbers of systematic reviews and meta-analyses have been published to synthesis the effectiveness of CM in recent years and often arrive at discordant conclusions. For example, Joo et al. [9] retrieved and synthesised results from nine experimental studies and found that CM effectively improved patients’ QoL and symptom management. While Aubin et al. [10] reported equivocal effect on both QoL and symptom management. Chan et al. [11] reported that four of the five randomized controlled trials showed insignificant impact of CM on patients’ QoL. The inconsistent evidence on the impact of CM may confuse or mislead the future case management development and relevant policy making. Considering the exist of several systematic reviews and research synthesis available to inform the application of case management for cancer patient care improvement, umbrella review could now be undertaken to compare and contrast published reviews and to highlight the consistent or contradictory findings around the effect of CM on manifold aspects of cancer patient care [12]. Thus, the current review was conducted to 1) synthesis systematic reviews that assess the effects of CM on cancer patient outcomes (e.g., QoL, functioning status, symptom management, satisfaction, etc.) and health care utilization outcomes (e.g., cost, hospital admissions, length of stay, treatment received compliance, etc.), 2) summarize measurement used in evaluating patient outcomes and health care utilization outcomes.
Methods
Design
This umbrella review followed the Joanna Briggs Institute (JBI) Umbrella Review (UR) methodology [12] and adhered to the Preferred Reporting Items for Overviews of systematic reviews (PRIO) checklist (see Additional file 1) [13]. This review has been registered with the Open Science Framework (https://doi.org/10.17605/OSF.IO/7YQAP).
Study searching methods
We performed literature search in five databases including MEDLINE (Ovid), EMBASE (Ovid), PsycINFO, CINAHL, and Scopus from inception to July 2022. Ethical approval and patient consent were not necessary since all analyses were based on previously published articles. The searching strategies in all five databases were developed with the help of a health science librarian. See Additional file 2 for the searching strategy and results in MEDLINE (Ovid). The studies were selected using the following inclusion and exclusion criteria.
Inclusion and exclusion criteria
Population
Individuals diagnosed with any type of cancer at any cancer stages (early to advanced). Reviews targeted on people with no specified cancer diagnose were excluded.
Intervention
Case management interventions targeted on cancer patients. Case management is defined as a “collaborative process of assessment, planning, facilitation, care coordination, evaluation, and advocacy for options and services to meet an individual’s and family’s comprehensive health needs through communication and available resources to promote quality, cost-effective outcomes” [8]. Only reviews in which the effectiveness of CM as defined above was analyzed separately from other interventions were considered.
Comparison
Individuals in comparison groups received “treatment as usual” (TAU). TAU may include various interventions called “standard of care,” “usual care,” or “standard treatment,” but generally refers to treatment as it is commonly provided. Only studies that compared case management with “TAU” were selected.
Outcomes
Patient outcomes (e.g., quality of life, symptom management, functioning status), health care utilization outcomes (e.g., cost, hospital admissions, length of stay), etc.
Setting
Acute care hospitals and primary care settings (e.g., long-term care, nursing homes, community care services). Hospital was defined as any department of internal medicine or surgery as well as unspecified hospital settings.
Study design
Systematic review/meta-analysis that only included quantitative studies. We excluded studies full-texts unavailable online.
Study selection
All retrieved studies were imported into Covidence systematic review software [14] and the duplicates were removed. Then, titles and abstracts were independently assessed by two researchers (XW and XD) according to the inclusion criteria. After that, the full texts of the selected abstracts were obtained and reviewed by the same two researchers (XW and XD) independently. The reference list of included studies was reviewed and searched for additional studies. Any disagreement between the two researchers were resolved through consultation with a senior researcher (PL).
Quality appraisal for included reviews
Two reviewers (NW and LM) independently assessed the methodological quality of the individual studies using the JBI Critical Appraisal Checklist for Systematic Reviews and Research Syntheses [15]. The tool aims to determine the extent to which the review has addressed the possibility of bias in its design, conduct and analysis [15]. It consists of 11 criteria scored as yes, no, unclear, or not applicable. We adopted a scoring system used in previously published systematic reviews [16, 17]. For each article, a rating score was derived by taking the number obtained in the quality rating and dividing it by the total number of possible points allowed, giving each manuscript a total quality rating between 0 and 1. Studies were then classified as low (0–0.25), low-moderate (0.26–0.50), moderate (0.51–0.75), or high (0.76–1.0).
Data extraction
We developed the data extraction form based on the research questions, and extracted following information: characteristics of included reviews such as publication year range, whether conducted meta-analysis or not, type of cancer patients, age of population, type and number of primary studies included; intervention names, components, and duration; outcomes and evaluation tools used; author’s conclusions and interpretations. Two researchers (NW and LM) extracted data independently from all included articles into an Excel spreadsheet and another researcher (XL) verified it for accuracy.
Data synthesis
We were unable to statistically pool outcomes due to the heterogeneity of outcomes of the included reviews. Therefore, we conducted a narrative synthesis [18] of the numerical data of individual studies outcomes. The studies were summarized and synthesised by two reviewers (NW and ZS) independently and double checked by a third author (HY). Following the JBI UR methodology [12], we used a summary table to present clear, specific, and structured results from the selected reviews, and then synthesised these results to identify broad conclusions. To summarized information about the interventions we coded data into features, components and delivery strategies, and inductively developed themes within each domain as they emerged from the studies. As suggested by Li and colleagues [19], we grouped outcomes into: global QoL of patients, functional status (i.e. physical, cognitive, emotional, role, social), symptom management, cost, hospital (re)admission, length of stay, treatment received compliance, provision of timely treatment.
For clarity the term ‘primary studies’ refers to the articles found within the included reviews. As several primary studies are included in more than one review, the overall results and conclusions of an overview can be biased. To assess this bias, the degree of overlap between reviews was calculated with the Corrected Covered Area (CCA) method. The details of the CCA calculation have been described by Pieper and colleagues [20] elsewhere. A CCA score of less than 5% is regarded as a slight overlap, 5–9.9% as moderate overlap, 10–14.9% as high overlap and over 15% as a very high level of overlap. This measure has been validated in which the number of overlapped primary publications has a strong correlation with the CCA [21].
Results
Search outcome
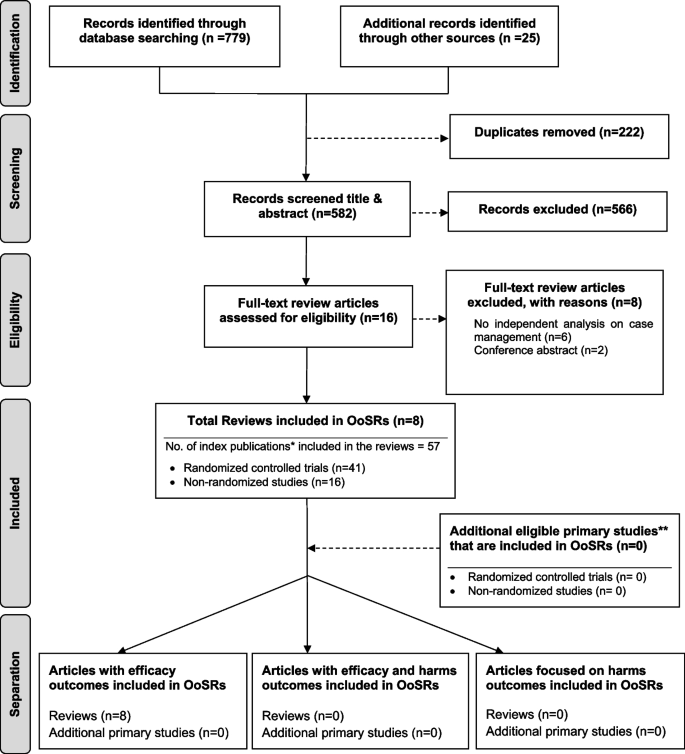
As shown in Fig. 1, our search strategy generated 804 potentially relevant records. Upon removing the duplicates, 582 studies screened by title and abstract, 16 were identified for full text screening. We excluded eight of the 16 studies for the following reasons: no independent analysis on the effect of case management (n = 6), or conference abstract (n = 2). The eight remaining systematic reviews were selected and assessed for methodological quality. In total, all the eight reviews included 57 primary studies, among which 12 were duplicated included in two or three reviews. Forty-one of the 57 primary studies were randomized controlled trials (see Additional file 3 for included primary studies).
Methodological quality assessment
The quality assessment scores are presented in Table 1. Only one review was rated as moderate because not clarify whether two or more reviewers independently assessed the quality of included primary studies, and did not report the methods to minimize errors in data extraction or publication bias. The other seven reviews were rated as high quality. Despite rated as strong, the seven reviews still companied with one or two issues on the assessment of heterogeneity, search strategy, and recommendations for policy and/or practice.
Characteristics of included studies
Table 2 presents a descriptive summary of characteristics of the eight systematic reviews [9,10,11, 19, 23,24,25,26]. The eight reviews aimed to identify evidence of the effectiveness of CM on cancer patients. Three of the studies were a systematic review with meta-analysis [10, 25, 26]. Five of the eight reviews adhered to the PRISMA statement [11, 19, 24,25,26], two adopted Cochrane systematic review methodology [9, 10].
The eight reviews were published between 2008 and 2021, the primary studies in the reviews were published between 1983 and 2018. The number of primary studies regarding to CM included in each review ranged from three to 20. Five of the eight reviews included only randomized controlled trials (RCTs), the remaining reviews included a combination of study designs that involved RCTs, quasi-experimental and non-experimental studies (e.g., cohort study). The age of review participants ranged from 7 to 97 years and mean ages range from 48.63 to 66.31 years, which covers populations from children to elders. The total number of participants in each review ranged from 327 to 9601. Seven of the eight reviews included primary studies targeted on multiple types of cancer including breast, lung, colorectal, cervical, ovarian, prostate, gastric, hepatocellular, etc. Most of the primary studies included in the eight reviews were conducted in the United States, and there were also studies conducted in Canada, Australia, Europe (i.e., Germany, UK, Turkey, Switzerland, Denmark, Switzerland, Sweden, Norway, Netherlands) and East Asia (i.e., Hong Kong, Taiwan, South Korea, and Malaysia).
CM interventions
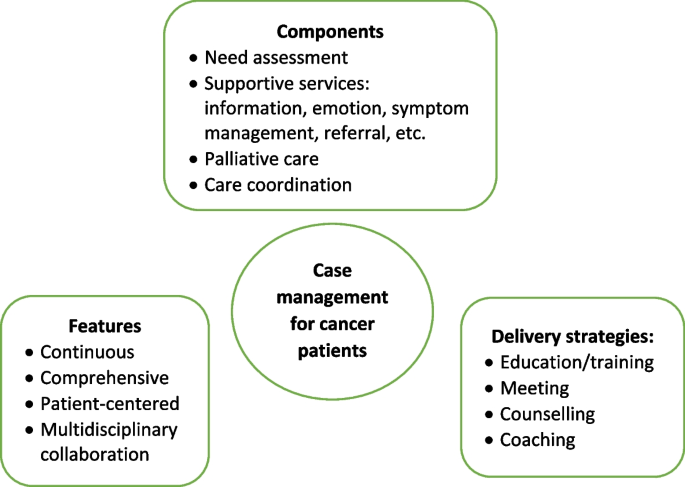
As shown in Table 2, three studies reviewed trials of nurse-led CM interventions [9, 25, 26], two reviewed CM-like interventions that not termed as ‘CM’ while meet the CM definition by the CMSA [8, 23, 24]. Only one study reviewed CM focus solely on skill-training or symptom management [19]. All studies reviewed trials that facilitated the CM in a multidisciplinary collaboration approach. The duration of CM ranged from 4 days to 5 years. We presented the feature, components and delivery strategies of CM interventions for cancer patients in Fig. 2 by summarizing descriptions in each review. Congruent with the components defined by CMSA [8], all CM interventions included patient assessment, supportive services such as information and emotion support, care coordination by conducting education, consultation, and in-person, telephone or online coaching for regular follow-up. One critical component of CM interventions for cancer patients is the provision of palliative care. Control groups (CGs) of all studies reviewed in the reviews received usual treatment of care.
Corrected Covered Area (CCA)
Table 3 presents the CCA for each outcome and as a whole. Overall, primary studies had a slight overlap across the eight reviews (CCA = 4.5%). In addition, no overlapping of primary studies was found for six of the 16 outcomes, including self-efficacy, psychological function, hospital (re)admissions, length of stay, and provision of timely treatment. Only one outcome (i.e., symptom management) showed slight overlap (0.7%). The CCA for other five outcomes (i.e., global QoL, physical function, role function, patient satisfaction, cost) evaluated by more than 2 reviews were between 5 to 9.9%, indicated a moderate overlap. The CCA for survivor status, cognitive function, emotional function, and treatment received compliance were over 10%.
Measurement used
Table 4 presents the quantitative measurement used in primary studies. As shown in Table 4, studies investigated global QoL using different QoL-related scales, among which Functional Assessment of Cancer Therapy (FACT) (used in 15 primary studies) were most frequently applied, followed by the European Organisation for Research and Treatment of Cancer Core Quality of Life Questionnaire 30 (EORTC QLQ-C30) (used in 11 primary studies), and short form health survey (i.e., SF-8, SF-12, SF-36) (used in 10 primary studies). Different types of FACT tool were used according to the cancer types. For example, FACT-G was used for general cancer patients assessment, and FACT-B was used to evaluate breast cancer-related QoL. For the assessment of overall symptom management, SF-36 and Symptom Distress Scale (SDS) were used most frequently (used in four primary studies each). Different dimensions of SF-36 were also applied to evaluate other outcomes such as physical, emotional, and social function. Hospital Anxiety and Depression Scale (HADS) was the top employed tool in measuring the psychological function of patients. Patients’ sick leave days and the number of patients return to work were top employed metrics to evaluate the role function of patients. No unified tools were utilized to assess patient satisfaction towards the CM and majority of the primary studies used self-developed questionnaires.
Effect of CM on patient and health care utilization outcomes
The main outcomes from the seven systematic reviews are presented and summarized in Table 5. Seven of the eight reviews reported the effects of case management on patients’ global QoL and showed mixed findings. Around half (49%, 19/39) of the primary studies included in the seven reviews reported significant positive impact of CM on global QoL. As for the functional status, there was a strong concordance among primary studies regarding the effectiveness of CM in improving cognitive function (e.g., uncertainty, health perceptions) (89%, 8/9); Equivocal effects were reported on psychological (e.g., patient anxiety, depression), physical (e.g., arm function), role function (e.g., sick leave days, patients returning to work), emotional (e.g., mood) and social function (e.g., social support) [9, 11, 26]. The findings regard to symptom management were more positive, with 75% (18/24) primary studies included in seven reviews revealed significant positive impact of CM on symptom severity and symptom distress decrease of pain, nausea, fatigue, discomfort, etc. Three of the four primary studies in two reviews [9, 11] showed no significant influence of CM on patients’ self-efficacy. Wulff et al. [23] and Aubin et al. [10] reported mixed findings on the impact of CM on survivor status, with four of the six primary studies reported significant positive impact. The effect of CM on patient satisfaction was reported in five reviews and showed mixed results.
Of the eleven primary studies reported cost, only one controlled before-and-after study in Joo et al.’s [9] review reported significant impact on monthly cancer-related medical costs. The evidence concerning patients’ length of stay yielded no significant findings. Overall significant positive effect was reported on hospital (re)admission (e.g., inpatient and ICU admission rate), treatment received compliance (e.g., therapy acceptance or completion rate), and provision of timely treatment.
Discussion
This umbrella review is the first to summarize the results of systematic reviews that synthesised the evidence on the effectiveness of CM on cancer patient outcomes and relevant health care utilization. Most reviews (7/8) showed a high methodological quality. Different tools were used to measure the effect of CM on the same outcome. The evidence regards to the effectiveness of CM is mixed. The summarized results revealed that CM was more likely to improve symptom management, cognitive function, hospital (re)admission, treatment received compliance, and provision of timely treatment for cancer patients. Overall equivocal effect was reported on cancer patients’ global QoL, psychological, physical, role, emotional and social function, self-efficacy, survivor status, and patient satisfaction.
No universal tools were used to measure improvement of each outcome in the CM group compared with the control group, making it challenging to conduct a meta-analysis of studies results [22, 27]. This is a common issue faced the included reviews. Five of the eight reviews failed to conduct meta-analysis due to the heterogeneity [9, 11, 19, 23, 24]. Joo and Huber [22] conducted a review of reviews on the effect of CM on health care utilization outcome of chronic illness patients, they recognized the same problem and suggested using valid and standardized tools to minimize the differences in measurements. Despite various tools used, our review showed that FACT, EORTC QLQ-C30, and short form health survey (i.e., SF 36, SF 12, and SF 8) were most frequently applied to measure the effect of CM on the global QoL of cancer patients. These tools were also used in evaluating specific dimensions of QoL such as psychological, physical, emotional, and social function. This aligned with previous reviews [28, 29] that found FACT and EORTC QLQ-C30 were the most common and well developed QoL instruments in cancer patients. FACT-G is considered appropriate for use with any types of cancer patients [30]. It is a 27-item tool that includes four primary QoL domains: physical well-being, social/family well-being, emotional well-being, and functional well-being [31]. Other versions of FACT (FACT-B [32], FACT-L [33] and FACT-E [34]) for specific type of cancer patients were developed by incorporating the four dimensions of FACT-G with additional cancer type-specific questions. EORTC QLQ-C30 was another type of QoL assessment tools for cancer patients specifically. It was developed by Aaronson et al. [35] and contains four domains: physical, emotional, cognitive and social functions, and a higher score indicates better QoL. The Short Form Health Survey is the most commonly used measure in evaluating QoL domains of patients suffering from a wide range of medical conditions [36]. Research found it provides reliable and valid indication of general health among cancer patients [37, 38].
QoL is the most frequently evaluated outcome in our review with 39 primary studies in seven reviews reported the global QoL of cancer patients. Joo et al. [9] found that CM interventions improved QoL of cancer patients. Yin and colleagues [24] revealed that cancer patients achieved better physical and psychological condition through symptom management, needs assessment, direct referrals, and other services in CM. However, summarized results in our review show that the CM had equivocal effect on cancer patients’ global QoL and dimensions including psychological, physical, role, emotional and social function. Cognitive function is the only dimension showed positive change. Despite CM interventions share similar definitions and principles [8]. It is hard to foresee which aspect(s) of CM interventions contribute to certain effects due to their comprehensiveness [24]. Yin et al. [24] argued that the control group may receive a higher quality treatment than planned usual care since all the participants were not blinded and they have been informed about the aim of the study. Indicating a more rigorous design and evaluation is needed to avoid this information bias.
In the meantime, included reviews claimed that few primary studies reported enough details about CM interventions, including model used [10, 11], dose and intensity [9, 19, 24], interventionist qualifications [11], protocol or manual used [9, 23], and fidelity [23]. Particularly, the COVID-19 pandemic has considerable influence on the care delivery for cancer patients. For example, the more frequently utilization of remote patient monitoring technologies that incorporate community resources, primary care and allied health disciplines, as well as clinics to keep cancer patients away from acute care hospitals as much as possible [39]. Many of these changes have been integrated within routine case management for cancer care during the pandemic [39]. It is well-needed to report how those CM intervention were conducted follow standard reporting guidelines, in order to provide recommendation for future research.
Our review showed that CM is likely to improve the symptom management. Eighteen of the 24 included primary studies reported positive effect of CM on symptom management, including decrease symptom distress or severity of fatigue, pain, nausea, and vomiting. The same positive effect on symptom management was also revealed in other types of patients. Joo and colleagues [40] found that CM reduced substance use and significantly influenced abstinence rates among populations experienced substance disorders. Reviews by Stokes et al. [27] and Welch et al. [41] revealed positive effect on symptom release among people with long-term conditions and diabetes patients, respectively. The multidisciplinary collaboration approach adopted [10], and availability of professional support post-hospitalization [9, 41] in CM might contribute to the improvement of symptom management. Specifically, multidisciplinary team involves physicians, nurses, and aligned healthcare professionals provides throughout and multifaced symptom assessment and management [10]. In addition, CM programs continuously follow up and advocate for patients’ concerns [8]. Specifically, case managers are available to patients 24 hours a day by phone call even after discharged, providing opportunity for immediate professional guidance on symptom management [9].
As for other patient outcomes, there is insufficient evidence of effect on self-efficacy and survivor status of cancer patients. Only three and four primary studies in total reported these two outcomes, respectively. Eleven primary studies in five reviews reported patient satisfaction and showed mixed results. Inconsistent results were found in a review of reviews by Buja et al. [7] which concluded strong evidence of CM improving satisfaction of patients with long term condition. In agreement with Joo and Huber’s [25] review, we found that CM favorably affect healthcare utilization outcomes such as treatment received compliance, hospital (re)admission, and provision of timely treatment. While the strength of the evidence was limited either by the high level of primary studies overlapping (CCA) (i.e., treatment received compliance, CCA = 13.3%) or the small number of studies reported certain outcomes (i.e., hospital admission, provision of timely treatment). Notably, the summarized results from included reviews conclude that despite theoretical benefits [8], in practice there is only slight evidence of benefits on reduction in the cost of care for cancer patients participated in CM interventions.
We provide some recommendations for future research based on the summarized results: 1) Future research should clearly describe details of CM intervention and its implementation, including theoretical underpinnings, dose and intensity, interventionist qualifications, protocol or manual used, fidelity, etc. In that way these details can be included in future systematic reviews, and effectiveness of individual elements of the intervention can be examined [27]. We recommend use standard guidelines to help organize the CM intervention reporting. For example, the Template for Intervention Description and Replication (TIDeiR) is one of the most popular guidelines that could be used to report the full breadth of CM interventions: from intervention rationale to assessments of treatment adherence and fidelity [42]. 2) More rigorous trials are needed to evaluate the effectiveness of CM. 3) Studies should also explore the barriers to and facilitators of CM implementation across various types of cancer patients at different stages, providing evidence for conducting successful CM implementation in the future.
Strengths and limitations
We conducted an umbrella review instead of a meta-analysis due to the heterogeneity of review outcomes. Although an umbrella review can only show the tendency or direction of the effect of CM rather than providing the magnitude or significance level of influence [12], the current evidence on the effect of CM in cancer patients was comprehensively summarized. There were some challenges when conducting the review. First, the quality of the umbrella reviews was greatly affected by the quality of the original reviews [12]. In this study, we confirmed that the quality of the original reviews were mostly high as assessed by the JBI Critical Appraisal Checklist [15]. Second, if the primary studies were included in several reviews, they may produce bias related to overlapping effects [20]. By calculating the CCA, we showed that 75% (12/16) of the individual outcomes had no to moderate overlapping of primary studies between included reviews, revealing that these results from each review were relatively independent. Cautious are needed on the summarized evidence regards to the effect of CM on survivor status, cognitive function, emotional function, and treatment received compliance because of the high overlapping (CCA > 10) between the reviews reported those outcomes.
There are limitations in our review. The first limitation concerns that the searching was limited to English-language articles and did not access unpublished papers. Second, as suggested by the JBI UR methodology [12], we did not assess the quality of evidence from included reviews, it increased the uncertainty of the review findings.
Conclusion
Effective CM aims to influence the health care delivery system in improving the health outcomes of cancer patients, enhancing their experience of health care, and reducing the cost of care. Our review found mixed effects of CM reported in cancer patient care. The summarized results revealed that CM was likely to improve symptom management for cancer patients. We also found CM has the tendency to enhance cancer patients’ experience of health care such as reducing hospital (re)admission rates, improving treatment received compliance and provision of timely treatment. Only slight evidence of benefits was reported on reducing the cost of care for cancer patients. Overall, more rigorous designed primary studies are needed to demonstrate the effects of CM on cancer patients and explore the elements of effective CM interventions.
Availability of data and materials
All data generated or analysed during this study are included in this published article and its supplementary information files.
Abbreviations
- CCA:
-
Corrected Covered Area
- CGs:
-
Control groups
- CM:
-
Case management
- CMSA:
-
Case Management Society of America
- EORTC QLQ-C30:
-
European Organization for Research and Treatment of Cancer Core Quality of Life Questionnaire 30
- FACT-B:
-
Functional Assessment of Cancer Therapy - Breast Cancer
- FACT-E:
-
Functional Assessment of Cancer Therapy- Esophagus
- FACT-G:
-
Functional Assessment of Cancer Therapy- General
- FACT-L:
-
Functional Assessment of Cancer Therapy Scale-Lung
- QoL:
-
Quality of life
- HADS:
-
Hospital Anxiety and Depression Scale
- JBI:
-
Joanna Briggs Institute
- PRISMA:
-
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- RCTs:
-
Randomized controlled trials
- SDS:
-
Symptom Distress Scale
- SF-8:
-
Medical Outcomes Study 8-item short form health survey
- SF12:
-
Medical Outcomes Study 12-item short form health survey
- SF-36:
-
Medical Outcomes Study 36-item short form health survey
- TAU:
-
Treatment as usual
References
Ferlay J, Ervik M, Lam F, Colombet M, Mery L, Piñeros M, et al. Global cancer observatory: Cancer today: International Agency for Research on Cancer; 2020. https://gco.iarc.fr/today.
Ferlay J, Ervik M, Lam F, Colombet M, Mery L, Piñeros M, et al. Global Cancer observatory: Cancer tomorrow: International Agency for Research on Cancer; 2020. https://gco.iarc.fr/tomorrow.
Quaresma M, Coleman MP, Rachet B. 40-year trends in an index of survival for all cancers combined and survival adjusted for age and sex for each cancer in England and Wales, 1971-2011: a population-based study. Lancet. 2015;385:1206–18. https://doi.org/10.1016/S0140-6736(14)61396-9.
Yabroff KR, Dowling EC, Guy GP, Banegas MP, Davidoff A, Han X, et al. Financial hardship associated with cancer in the United States: findings from a population-based sample of adult cancer survivors. J Clin Oncol. 2016;34:259–67. https://doi.org/10.1200/JCO.2015.62.0468.
Yan AF, Stevens P, Holt C, Walker A, Ng A, McManus P, et al. Culture, identity, strength and spirituality: a qualitative study to understand experiences of African American women breast cancer survivors and recommendations for intervention development. Eur J Cancer Care (Engl). 2019;28:e13013. https://doi.org/10.1111/ecc.13013.
Yabroff K, Mariotto A, Tangka F. Annual report to the nation on the status of Cancer, part II: patient economic burden associated with Cancer care. J Natl Cancer Inst. 2021;113:1670–82. https://doi.org/10.1093/jnci/djab192.
Buja A, Francesconi P, Bellini I, Barletta V, Girardi G, Braga M, et al. Health and health service usage outcomes of case management for patients with long-term conditions: a review of reviews. Prim Heal Care Res Dev. 2020;21:1–21. https://doi.org/10.1017/S1463423620000080.
Case Management Society of America. CMSA’s standards of practice for case management, Revised 2016. 2016. http://www.naylornetwork.com/cmsatoday/articles/index-v3.asp?aid=400028&issueID=53653.
Joo JY, Liu MF. Effectiveness of nurse-led case Management in Cancer Care: systematic review. Clin Nurs Res. 2019;28:968–91. https://doi.org/10.1177/1054773818773285.
Aubin M, Giguère A, Martin M, Verreault R, Fitch MI, Kazanjian A, et al. Interventions to improve continuity of care in the follow-up of patients with cancer. Cochrane Database Syst Rev. 2012:1–193. https://doi.org/10.1002/14651858.CD007672.pub2.
Chan RJ, Teleni L, McDonald S, Kelly J, Mahony J, Ernst K, et al. Breast cancer nursing interventions and clinical effectiveness: a systematic review. BMJ Support Palliat Care. 2020;10:276–86. https://doi.org/10.1136/bmjspcare-2019-002120.
Aromataris E, Fernandez R, Godfrey C, Holly C, Khalil H, Tungpunkom P. JBI manual for evidence synthesis. In: Aromataris E, Munn Z, editors. JBI Manual for Evidence Synthesis. JBI; 2020. https://doi.org/10.46658/JBIMES-20-11.
Bougioukas KI, Liakos A, Tsapas A, Ntzani E, Haidich AB. Preferred reporting items for overviews of systematic reviews including harms checklist: a pilot tool to be used for balanced reporting of benefits and harms. J Clin Epidemiol. 2018;93:9–24. https://doi.org/10.1016/j.jclinepi.2017.10.002.
Veritas Health Innovation. Covidence systematic review sofware. Covidence. 2016. https://get.covidence.org/systematic-review-software?campaignid=15030045989&adgroupid=130408703002&gclid=EAIaIQobChMIvc7KuJDO-QIVh97ICh1BeQKnEAAYASAAEgI4APD_BwE.
Aromataris E, Fernandez R, Godfrey C, Holly C, Kahlil H, Tungpunkom P. Summarizing systematic reviews: methodological development, conduct and reporting of an umbrella review approach. Int J Evid Based Heal. 2015;13:132–40 http://joannabriggs.org/research/critical-appraisal-tools.html.
Gifford W, Rowan M, Dick P, Modanloo S, Benoit M, Al AZ, et al. Interventions to improve cancer survivorship among indigenous peoples and communities : a systematic review with a narrative synthesis. Support Care Cancer. 2021;29:7029–48. https://doi.org/10.1007/s00520-021-06216-7.
Gifford W, Squires J, Angus D, Ashley L, Brosseau L, Craik J, et al. Managerial leadership for research use in nursing and allied health care professions : a systematic review. Implement Sci. 2018;13:1–23. https://doi.org/10.1186/s13012-018-0817-7.
Popay J, Roberts H, Sowden A, Petticrew M, Arai L, Rodgers M, et al. Guidance on the conduct of narrative synthesis in systematic reviews: a product from the ESRC methods Programme. Lancaster: Lancaster University; 2006. https://www.lancaster.ac.uk/media/lancaster-university/content-assets/documents/fhm/dhr/chir/NSsynthesisguidanceVersion1-April2006.pdf.
Li Q, Lin Y, Liu X, Xu Y. A systematic review on patient-reported outcomes in cancer survivors of randomised clinical trials: direction for future research. Psychooncology. 2014;23:721–30. https://doi.org/10.1002/pon.3504.
Pieper D, Antoine SL, Mathes T, Neugebauer EAM, Eikermann M. Systematic review finds overlapping reviews were not mentioned in every other overview. J Clin Epidemiol. 2014;67:368–75. https://doi.org/10.1016/j.jclinepi.2013.11.007.
Choi J, Lee M, Lee JK, Kang D, Choi JY. Correlates associated with participation in physical activity among adults: a systematic review of reviews and update. BMC Public Health. 2017;17:1–13. https://doi.org/10.1186/s12889-017-4255-2.
Joo JY, Huber DL. Case management effectiveness on health care utilization outcomes: a systematic review of reviews. West J Nurs Res. 2019;41:111–33. https://doi.org/10.1177/0193945918762135.
Wulff CN, Thygesen M, Søndergaard J, Vedsted P. Case management used to optimize cancer care pathways: a systematic review. BMC Health Serv Res. 2008;8:1–7 http://www.biomedcentral.com/1472-6963/8/227.
Yin YN, Wang Y, Jiang NJ, Long DR. Can case management improve cancer patients quality of life?: a systematic review following PRISMA. Medicine (Baltimore). 2020;99:1–7. https://doi.org/10.1097/MD.0000000000022448.
Wu YL, Padmalatha KMS, Yu T, Lin YH, Ku HC, Tsai YT, et al. Is nurse-led case management effective in improving treatment outcomes for cancer patients? A systematic review and meta-analysis. J Adv Nurs. 2021;00:1–11. https://doi.org/10.1111/jan.14874.
McQueen J, McFeely G. Case management for return to work for individuals living with cancer: a systematic review. Int J Ther Rehabil. 2017;24:203–10. https://doi.org/10.12968/ijtr.2017.24.5.203.
Stokes J, Panagioti M, Alam R, Checkland K, Cheraghi-Sohi S, Bower P. Effectiveness of case management for “at risk” patients in primary care: a systematic review and meta-analysis. PLoS One. 2015;10:e0132340. https://doi.org/10.1371/journal.pone.0132340.
Lemieux J, Goodwin PJ, Bordeleau LJ, Lauzier S, Théberge V. Quality-of-life measurement in randomized clinical trials in breast cancer: an updated systematic review (2001-2009). J Natl Cancer Inst. 2011;103:178–231. https://doi.org/10.1093/jnci/djq508.
Montazeri A. Health-related quality of life in breast cancer patients: a bibliographic review of the literature from 1974 to 2007. J Exp Clin Cancer Res. 2008;27:1–31 https://jeccr.biomedcentral.com/articles/10.1186/1756-9966-27-32.
Cella DF, Tulsky DS, Gray G, Sarafian B, Linn E, Bonomi A, et al. The functional assessment of cancer therapy scale: development and validation of the general measure. J Clin Oncol. 1993;11:570–9. https://doi.org/10.1200/JCO.1993.11.3.570.
Webster K, Cella D, Yost K. The F unctional a ssessment of C hronic I llness T herapy (FACIT) measurement system: properties, applications, and interpretation. Health Qual Life Outcomes. 2003;1:79. https://doi.org/10.1186/1477-7525-1-79.
Brady MJ, Cella DF, Mo F, Bonomi AE, Tulsky DS, Lloyd SR, et al. Reliability and validity of the functional assessment of cancer therapy- breast quality-of-life instrument. J Clin Oncol. 1997;15:974–86. https://doi.org/10.1200/jco.1997.15.3.974.
Cella DF, Bonomi AE, Lloyd SR, Tulsky DS, Kaplan E, Bonomi P. Reliability and validity of the functional assessment of cancer therapy-lung (FACT-L) quality of life instrument. Lung Cancer. 1995;12:199–220. https://doi.org/10.1016/0169-5002(95)00450-F.
Darling G, Eton DT, Sulman J, Casson AG, Cella D. Validation of the functional assessment of cancer therapy esophageal cancer subscale. Cancer. 2006;107:854–63. https://doi.org/10.1002/cncr.22055.
Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, et al. The European organization for research and treatment of cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85:365–76. https://doi.org/10.1093/jnci/85.5.365.
Ware J, Kosinski M, Dewey J. How to score version two of the SF-36® health survey. Lincoln: QualityMetric Incorporated; 2000.
Treanor C, Donnelly M. A methodological review of the short form health survey 36 (SF-36) and its derivatives among breast cancer survivors. Qual Life Res. 2015;24:339–62. https://doi.org/10.1007/s11136-014-0785-6.
Lins L, Carvalho FM. SF-36 total score as a single measure of health-related quality of life: scoping review. SAGE Open Med. 2016;4:1–12. https://doi.org/10.1177/2050312116671725.
Chan RJ, Crawford-Williams F, Crichton M, Joseph R, Hart NH, Milley K, et al. Effectiveness and implementation of models of cancer survivorship care: an overview of systematic reviews. J Cancer Surviv. 2021:1–25. https://doi.org/10.1007/s11764-021-01128-1.
Joo J, Huber D. Community-based case management effectiveness in populations that abuse substances. Int Nurs Rev. 2015;62:536–46. https://doi.org/10.1111/inr.12201.
Welch G, Garb J, Zagarins S, Lendel I, Gabbay RA. Nurse diabetes case management interventions and blood glucose control: results of a meta-analysis. Diabetes Res Clin Pract. 2010;88:1–6. https://doi.org/10.1016/j.diabres.2009.12.026.
Hoffmann TC, Glasziou PP, Boutron I, Milne R, Perera R, Moher D, et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ. 2014;348:g1687. https://doi.org/10.1136/bmj.g1687.
Acknowledgements
Not applicable.
Dual (co-)authorship
We declared that no author has authored one or more of the included systematic reviews.
Funding
This study was supported by 1) Hunan Provincial Key Laboratory of Nursing (2017TP1004, PI: Jia Chen), Hunan Provincial Science and Technology Department, 2) Changsha Natural Science Foundation (kq2202365, PI: Nina Wang) Changsha Science and Technology Department, and 3) Management research foundation of Xiangya Hospital (2021GL12, PI: Nina Wang).
Author information
Authors and Affiliations
Contributions
All authors have contributed to the production of this review. NW and WC conceptualized and designed the study and are the guarantor of the paper. JC and ZS conducted the literature search. PL, XW and XD were involved in the study screening. NW, LM and XL participated in the quality appraisal and data extraction. NW, ZS and HY conducted the data analysis. NW drafted the manuscript. WC and XL revised the manuscript. All authors participated in the review of the manuscript and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Wang, N., Chen, J., Chen, W. et al. The effectiveness of case management for cancer patients: an umbrella review. BMC Health Serv Res 22, 1247 (2022). https://doi.org/10.1186/s12913-022-08610-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12913-022-08610-1